- How Staffing Platforms Improve Compliance in Healthcare
- Clinical Research in Western Europe: How Belgium and France Are Setting New Standards
- OEM Toll Processing Elevates High-Value Products | By Jeff Elliott
- HeartBeam Advances Patient Convenience with At-Home ECG Technology
- Patient Trust: The New Cornerstone of Healthcare Marketing
- Philips Launches Transcend Plus for EPIQ CVx and Affiniti CVx, Delivering Breakthrough Image Quality and FDA-cleared AI Enhancements In Cardiovascular Ultrasound
- iXCells Biotechnologies Selected to Perform the First Pilot in Patient-Led ALS Precision Medicine Effort
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
CorVista Health Presents New Data on Non-Invasive Point of Care Testing for Pulmonary Capillary Wedge Pressure (PCWP) Elevation Using Machine Learning
The poster titled ‘Point-of-Care Testing for Pulmonary Capillary Wedge Pressure Elevation Using Machine Learning on Non-invasive Signals’ was presented at the American College of Cardiology’s Annual Scientific Session (ACC.25) in Chicago on March 30, 2025.
MedCognition Announces Extended Reality (XR) Medical Simulation Training Technology Receives Broad Method Patent from USPTO
Integrated within the company’s PerSim® medical education and training platform, the patented XR-based technology increases training efficacy by providing a safe and controlled environment that closely resembles authentic patient encounters, allowing students to refine their clinical skills and improve preparedness through real-world scenario simulations.
Myant Launches Care360: AI-Powered Precision Cardiac Assessments Delivered Directly to Patients and Physicians
“There has been far too little effort to raise awareness about atrial fibrillation—the most common arrhythmia and a leading driver of stroke, heart failure, and mortality,” said Dr. Yaariv Khaykin, Chief Medical Officer of Myant. “Atrial Fibrillation leads to impaired quality of life and affects productivity, yet many patients don’t even realize they have it. Some experience palpitations, others just feel fatigued and attribute it to aging. Many physicians remain unaware of innovations in Atrial Fibrillation management that could transform care. Myant Care360, with direct Atrial Fibrillation assessment referrals, provides unprecedented access to specialized care and early intervention—strategies that have been shown in randomized studies to slow or even reverse the progression of Atrial Fibrillation.”
CharmHealth Elevates Patient Care With Charm AI Scribe
Charm AI Scribe captures the natural dialogue between the patient and provider during a visit and transcribing it in real time.
Clinical Trials
Mabwell Receives NMPA Approval for Clinical Trial of Novel Nectin-4 Targeting ADC in TNBC
The study includes two cohorts: Cohort A will enroll patients with locally advanced or metastatic TNBC who have previously received taxane/anthracycline-based chemotherapy and topoisomerase inhibitor based antibody-drug conjugate treatment, and will receive 9MW2821 monotherapy; Cohort B will enroll patients with locally advanced or metastatic TNBC who have not previously received systemic therapy, and will receive a combination treatment of 9MW2821 and a PD-1 inhibitor reports Mabwell.
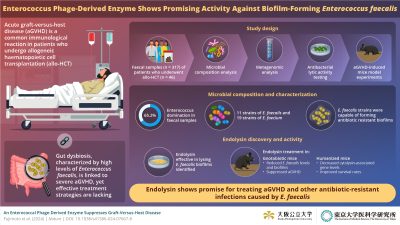
Phage-Derived Enzyme Targets E. faecalis Biofilms to Mitigate Acute Graft-Versus-Host Disease
In a recent study published in the journal Nature, our researchers have identified a bacteriophage-derived enzyme called endolysin that targets Enterococcus faecalis biofilms. This discovery offers a promising new approach to mitigating acute graft-versus-host disease (aGVHD) in patients undergoing stem cell transplantation. The enzyme’s ability to specifically target harmful bacteria without affecting beneficial ones could revolutionize treatments for aGVHD.
OSE Immunotherapeutics Announces Publication of Preclinical Efficacy Results with Lusvertikimab in Acute Lymphoblastic Leukemia in the Journal ‘Blood’
Nicolas Poirier, Chief Executive Officer of OSE Immunotherapeutics, concludes: “We are very pleased with this publication on Lusvertikimab in ‘Blood’, a high-level journal within the field of hematology whose manuscripts are reviewed by prominent specialists. Novel targeted immunotherapy options are urgently needed for B-ALL and T-ALL patients and we are happy to collaborate with the research leaders in hematology from the University of Kiel to face this clinical challenge.”
Biotechnology News
Lonza Launches Innovaform™ Accelerator, the New Innovation and Formulation Center in Colmar (FR)
Christian Seufert, President, Capsules & Health Ingredients, Lonza, commented: “The Innovaform™ Innovation and Formulation Accelerator represents a unique solution for drug developers seeking to solve complex dosage form and delivery challenges through innovation.
NanoVation Therapeutics Announces Multi-Target Partnership with Novo Nordisk to Develop Genetic Medicines Targeting Cardiometabolic and Rare Diseases
Under the terms of the agreement, Novo Nordisk and NanoVation Therapeutics will collaborate on two lead programs to develop base-editing therapies for certain rare genetic diseases, and up to five additional future targets for cardiometabolic and rare diseases.
Circular Genomics Announces Launch of MindLight, the 1st SSRI Antidepressant Response Test Using Brain-Derived Biomarkers
MindLight is the first circular RNA biomarker-based assay designed to predict whether a patient with depression, also known as major depressive disorder, will respond to selective serotonin reuptake inhibitor (SSRI) antidepressant treatment.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

A Strategic, Compliant Response to No Surprise Act Supported by Medical Billing Partnership | By Christine Cooper, CEO of aequum LLC
More than a year ago, the U.S. healthcare system marked a turning point with the passage of the No Surprises Act (NSA). Signed into federal law after extensive negotiation between health plan insurers and providers, the NSA took full effect at the start of 2022 to protect the rights of patients and health plan participants from receiving excessive balance bills for certain emergency services and from out-of-network providers at in-network facilities.

The Evolution of Mixed-Modes Patient-Reported Outcomes Guidance: A Journey Towards Evidence-Based Practices in BYOD | By: Dr. Jill Platko, Vice President of Scientific Services at Suvoda
Collecting patient-reported outcome (PRO) data is an integral function of clinical research, providing the patient perspective that enriches the evaluation of treatment effectiveness. Historically, patient-reported outcomes measurement (PROM) was performed predominantly with paper-based assessments. However, over the past two decades, there has been a notable paradigm shift towards the electronic collection of data.

Cryoablation Therapy for Breast Cancer (and other cancers) | By Dr. John Oertle, Chief Medical Director at Envita Medical Centers
Cancer care continues to be one of the key cost drivers contributing to the $3.5 trillion healthcare costs in the United States, but minimally invasive treatments like Cryoablation Therapy may hold the promise of significantly reducing oncology related costs, while improving patient outcomes. It has the potential of reducing months of recovery time to only a few minutes, and the scope of cryoablation expands exponentially especially when utilized in concert with personalized precision medicine. Read on.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding

Vicarious Surgical Raises An Additional $13.2 Mil in Capital
Vicarious Surgical added Philip Liang and Ric Fulop to their Board of Directors.
Nalu Medical Announces $65 Million Equity Financing to Advance Treatment for Chronic Neuropathic Pain
“Nalu Medical has made tremendous strides commercially and clinically in the past two years. Novo’s investment in Nalu reflects that progress and the confidence they share in the strong commercial potential for Nalu,” observed Geoff Pardo of Gilde Healthcare and Chairman of the Nalu Board.

Neurolief Announces Strategic Equity Investment by Sawai Group Holdings
Mitsuo Sawai, CEO of Sawai Group Holdings, highlighted their commitment to improving patient care and expanding treatment options for migraine and depression through the introduction of Relivion® for at-home treatment under doctor supervision.

LUMA Vision Announces $22 Million in New Financing to Secure FDA Approval and Advance Development of 4D Cardiac Imaging and Navigation Platform
The Series A3 financing added three new investors comprising an undisclosed multinational strategic investor, Atlantic Bridge Growth Fund and Bayern Kapital, and was led by existing investors EQT Lifesciences, ABV Uni Fund and imec.xpand. The new funding adds to prestigious grants won earlier by LUMA Vision from the Irish government’s Disruptive Technologies Innovation Fund ($6 million) and the European Union’s EIC Accelerator ($3 million).
Market Reports
Advancements In Imaging

Techsomed Announces Field Evaluation of its Image Guided Ablation Therapy Software Using GE HealthCare LOGIQ™ Ultrasound Systems at Top US Clinical Sites
Techsomed’s BioTrace platform, currently in advanced clinical evaluation, is a significant advancement in Image Guided Ablation Therapy. The technology leverages standard imaging—such as ultrasound (US) along with computed tomography (CT) or magnetic resonance imaging (MRI).

GE HealthCare Advances PET/MR Capabilities with AIR Technologies
Jie Xue, President and CEO of MR at GE HealthCare. “Our latest PET/MR technology will directly impact the most challenging diseases, such as prostate cancer and Alzheimer’s Disease. These diseases require precise and comprehensive imaging for accurate diagnosis, treatment planning, and therapy monitoring. We are excited to see SIGNA PET/MR AIR addressing these challenges and fulfilling our vision of providing access to advanced, personalized care.”

Jun 01, 2023 Philips and Masimo Introduce New, Advanced Monitoring Capabilities to Philips High Acuity Patient Monitors
“Combining our expertise in noninvasive monitoring and signal processing technologies with Philips’ expertise in integrated patient monitoring and therapy solutions is a win-win for patients and clinicians alike,” said Jon Coleman, President of Worldwide OEM Sales and Global Health, Masimo. “We are proud that Philips has chosen to make our innovative SedLine, O3, and NomoLine technologies available to their customers. We look forward to continuing our partnership with a focus on improving patient outcomes and reducing the cost of care.”
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

Adapting to the “New Normal”: Timur Yusufov Discusses Building Resilient Healthcare Services Post-Pandemic
Timur Yusufov explains the importance of developing resilient healthcare services post-pandemic and how this can be achieved with the right strategies and new technological advancements.

Maximizing Healthcare IT Consulting for Streamlined Operations
Some outside the information technology community fear that the increasing use of computers in healthcare will unnecessarily complicate patient care. However, it’s the goal of

The Secrets of Dental Office Upgrades: 6 Tips for a Thriving Practice
The dental industry has evolved significantly over the years, with advancements in technology, patient expectations, and the competitive landscape pushing dental practitioners to constantly improve

Impact of Technology on Medical Advancements
When technology and medicine come together, it means that in the future, diseases will be found early and treated more effectively. Care for patients is getting better and better because of this vital relationship.
Healthcare Careers
Nurses Corner

Top 5 Nursing Resume Mistakes to Avoid
As a professional nurse, you know the importance of attention to detail and thoroughness in your work. These same principles should also apply to your

6 Questions to Ask Your Recruitment Agency
A recruitment agency can be a valuable resource for companies looking to hire new employees. However, employers must understand how these agencies work and what

5 Important Tips to Prepare for NCLEX-RN Exam
Preparing for a major exam such as the NCLEX-RN can be a nerve-wracking experience. Having the right knowledge and skills to prepare for the exam