- SMSbiotech Initiates Groundbreaking Phase 1 Human Clinical Trial for COPD
- Owlet Achieves TGA Certification for Dream Sock™
- Method AI Raises $20M in Series A Financing
- The Business of Bringing Medical Devices to Market
- See Beyond the Surface: How Cellvizio® Redefines Accuracy for Detecting and Treating Cancers | Byline by Daryl C. Donatelli, MBA, President, U.S. & Head of Global Marketing, Mauna Kea Technologies
- Stable Bills, Faster Cash
- Pleural Dynamics Announces Completion of Enrollment of “The ACES Study for Aseptic Pleural Effusions,” a Post Market Study Evaluating the Novel ACES Automatic Continuous Effusion Shunt System
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
BiVACOR Total Artificial Heart Successfully Implanted in First Patient in Australia; TAH in Place Over 100 Days
Following weeks of in-hospital monitoring, the patient was the first in the world to be discharged from the hospital with the BiVACOR Total Artificial Heart as he awaited a heart transplant. After having the device for 105 days, a donor heart became available, and the patient received a heart transplant on March 6, 2025.

What Should Pharmacies Know About Child-Resistant and Senior-Friendly Blister Packaging: Key Considerations and Best Practices
Implementing child-resistant and senior-friendly blister packaging is essential for pharmacies to enhance safety and accessibility. Such packaging significantly reduces the risk of accidental poisoning among children by making it difficult for them to open, while remaining easy for seniors to use.
Innovaderm Research Rebrands as Indero | a Dual-Focus CRO
Indero is a dual-focus CRO for dermatology and rheumatology, with over 25 years of experience in clinical research and trial delivery.
Reflow Medical Expands Global Reach with New European Subsidiary in Landsberg am Lech, Germany
Isa Rizk, Co-Founder and CEO of Reflow Medical. “This presence allows us to better address the needs of international markets by providing direct sales solutions and forming key distribution collaborations. Our goal is to deliver breakthrough technologies that support physicians and improve patient outcomes worldwide.”
Clinical Trials

Venus Medtech VenusP-Valve Completed First Implantation in IDE Pivotal Clinical Study in U.S.
The VenusP-Valve PROTEUS STUDY, abbreviated from Evaluation of the PerfoRmance Of The VEnUsP-Valve System in Patients with Native Right Ventricular Outflow Tract (RVOT) Dysfunction, is a prospective multi-center non-randomized interventional study in patients with RVOT disorders comorbid with moderate or greater pulmonary regurgitation. With a target enrollment of 60 subjects, data from this trial will support VenusP-Valve’s registration with the U.S. FDA and Japanese Pharmaceuticals and Medical Devices Agency (PMDA).
Pfizer Reports | ELREXFIO ™ Shows Median Overall Survival of More Than Two Years in People with Relapsed or Refractory Multiple Myeloma
“These compelling overall survival data support the clinical benefit ELREXFIO has already demonstrated and its potential to be a transformative treatment option for people with multiple myeloma,” said Roger Dansey, M.D., Chief Development Officer, Oncology, Pfizer. “The latest results from MagnetisMM-3 reinforce the very promising efficacy observed with ELREXFIO in a relapsed or refractory setting, with deep and durable responses and although definitive conclusions cannot be drawn across studies, the longest reported median progression-free survival among B-cell maturation antigen bispecific antibodies.”
Updated Phase 2 CAPTIVATE Study Results
“After more than five years, the CAPTIVATE study findings confirm the sustained benefit of the fixed duration combination of ibrutinib and venetoclax as a first-line treatment for patients living with CLL, including in those with higher risk genomic features,” said Paolo Ghia MD, Università Vita-Salute San Raffaele and IRCCS Ospedale San Raffaele, Milan, Italy, study investigator.‡ “This all-oral, chemotherapy-free, fixed-duration regimen offers eligible patients the advantage of an extended, treatment-free interval while effectively keeping their disease under control.”
Biotechnology News
WuXi Biologics Successfully Completes First Scale-Up of High-Productivity Bioprocessing Platform WuXiUI™ in 2,000L GMP Manufacturing
By leveraging its ultra-intensified fed-batch platform, WuXiUI™, WuXi Biologics has completed its first scale-up to 2,000L drug substance (DS) GMP manufacturing, achieving a 4-fold productivity improvement compared to the traditional fed-batch process. The competitive performance achieved through the utilization of both WuXiUI™ and the proprietary platform cell culture media MagniCHO™ led to significant reduction in overall DS manufacturing COGS
The consistent performance of WuXiUI™ – from small scales to 2,000L GMP manufacturing – is a testament to the advancement of the technology as a mature and robust platform capable of significantly improving the cost-effectiveness of biologics production

PCI Pharma Services In Bedford, New Hampshire Location Successfully Completes International Coalition of Medicines Regulatory Authorities Inspection
The new ICMRA program is designed to abbreviate the time necessary to receive regulatory approvals from multiple countries. Regulatory agencies from several countries can convene for one inspection as a team, allowing CDMOs such as PCI Pharma Services to attain approval from each of the participating ICMRA countries simultaneously rather than undergo separate, phased inspections.
Partillion Bioscience Announces Early Access Program for Nanovial Multicell Assays to Enable the Study of Cell-to-Cell Interactions
“Our Nanovial Multicell Assays are a game-changer, opening up new possibilities in research areas where the precise colocalization of cells is essential for studying complex cell-to-cell interactions or screening for therapeutic molecules released by one cell that act on another” said Joe de Rutte, Co-founder and CEO of Partillion. “By enabling these complex screens, all using standard life science instrumentation, we’re enabling scientists to scale studies of how cells interact with other cells, whether it’s better understanding tumors in the context of immune cells in their microenvironment, host-pathogen interactions or developing the next-generation therapies.”
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Transforming Hammertoe Procedures: Forma Medical’s OptimalHT Revolutionizes MIS Hammertoe Arthrodes | By Andrew C. Davison
As the President and CEO of Forma Medical, I am excited to share how our groundbreaking approach, known as OptimalHT, is set to revolutionize hammertoe treatment. Our mission at Forma Medical has always been clear: to enhance the lives of patients by providing innovative surgical solutions. Today, I want to take you on a journey through the challenges of hammertoe deformities, the birth of OptimalHT, its key benefits, and our commitment to advancing surgery.

A Platform Approach: Expanding a Molecular Diagnostic Device Beyond Human Applications | By Shaun Holt, CEO, Alveo Technologies
Holt writes, “Avian flu is a global crisis, and not just for the poultry industry. There are serious potential implications for human health as well. But, let’s start with agriculture. Some strains of avian flu can be highly pathogenic. Once a single bird shows symptoms the clock starts ticking — it’s not unusual for an entire flock of tens of thousands of birds to die within two to three days.”

The Hollywood Writers’ Strike and Ethical AI | By Ed Watal, Founder & Principal — Intellibus
Watal writes, “Less than 12 months ago, with the debut of AI-powered ChatGPT, the true power of AI hit the mainstream. Since then, it has established an unshakeable belief in everyone’s minds that artificial intelligence is here to stay and will forever alter a number of industries.” What do you think? Read on.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding

Cromatic Closes $5.3 Million in Oversubscribed Seed Round to Modernize Life Science R&D Outsourcing
Ann Lin, co-founder and CEO of Cromatic said, “Outsourcing R&D is one of the most powerful ways for innovative biotech companies to get off the ground. While founders of tech giants like Apple and Google could build their products in garages with minimal resources, founders in biotech are confronted with the capital expense of lab space and equipment and the lengthy process of building the right team before they can even test their ideas. That’s why we are building the most powerful R&D outsourcing platform for biotechs, so that scientists will have clarity, confidence and choice when evaluating and managing their service providers. Ultimately, we want to enable biotech companies to start in garages, just like tech giants.”
Access Vascular to Ramp Production With $22 Million Series C Financing
The financing will support a substantial production capacity increase for the unique MIMIX™ hydrophilic biomaterial vascular access devices developed by AVI, which are designed to evade the foreign body reaction by mimicking the human body’s natural chemistry, as well as further research into the potential for MIMIX to lower catheter infection rates, and expansion of AVI’s product portfolio notes Access Vascular.
Neteera raises an additional $6.7M Series B Extension
Neteera raised the first $13 million in April 2023 in a round led by Aescuvest, which led this extension as well. Foxconn Technology joined.
Sigrid Therapeutics Secures $4 Million in Oversubscribed Funding Round to Tap Into Booming Obesity and Diabetes Market
Sigrid Therapeutics notes the funds will be instrumental in accelerating the commercialisation of SiPore® across diverse markets, including medical devices, food supplements, veterinary, and oral health.
Market Reports
Advancements In Imaging
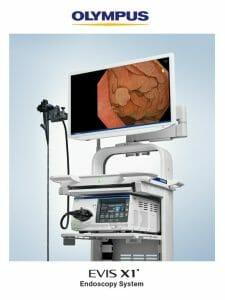
Olympus Set to Demonstrate Newest Endoscopy System at DDW
Olympus advises the EVIS X1 endoscopy system recently received FDA 510(k) clearance along with two compatible gastrointestinal endoscopes: the GIF-1100 gastrointestinal videoscope indicated for use within the upper digestive tract and the CF-HQ1100DL/I colonovideoscope indicated for use within the lower digestive tract, which will also be displayed.
Varex Launches XRD 3131N X-Ray Detector for High Throughput Electric Vehicle Battery Inspection
XRD 3131N X-Ray Detector enables a rapid and more thorough inspection process.
Health Check Bus: Lung Screening Project in Taiwan Reaches 8,000 Patient Mark
More than 8,000 patients have already been imaged by the Health Check Bus in Taiwan. The project started in July 2022 in Yunjianan and Penghu, an area comprising the county of Yunlin, Chiayi, Penghu and Tainan city. It is a cooperation between InnoCare Optoelectronics and the College of Medicine at National Cheng Kung University (NCKU) and focuses on mobile X-ray lung screening.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

How to Ensure Your Website Is ADA Compliant and Accessible
It is well known that accessible parking spots and ramps were made possible thanks to the enactment of the Americans with Disabilities Act (ADA). Because

Mastering the Lab: The Essential Guide to Lab Equipment Services
In this article, we delve deep into the intricate maze of these services and unearth their true value.

Community Care Impact: Making A Difference With Doctor Jobs
In this article, you’ll explore the significance of community care, the profound difference doctor jobs make, and why it matters to medical specialists and the medical device industry.

Choosing the Best Medical Billing Company: Specialized or General Solutions?
Introduction: Selecting the right medical billing company is a critical decision for healthcare practitioners seeking efficient revenue cycle management. When it comes to specialties like
Healthcare Careers
Nurses Corner

Seven Self-care Tips for New Nurses
After going through years of caffeine-fueled nights and late study sessions, you finally clear your board exams and get those letters prefixed with your name.

A Guide On How To Prepare For Nursing School
Are you thinking about becoming a nurse? If so, you will need to prepare for nursing school. This can be daunting, but with the right
American Academy of Nursing Releases Position Statement on Firearm Safety and Violence Prevention
The American Academy of Nursing has long supported policies to reduce firearm violence. Firearm violence, from suicides to mass shootings, is an epidemic in the United States, and as such, a public health approach to addressing the causes and risk factors leading to violence is necessary to safeguard the health of the nation.




