- What Are ELISAs? 3 Things to Know
- CGT Global Welcomes Dr Jerome Ritz to Its Board of Advisors | Enriching Knowledge of Cellular Therapies and Hematology
- What Are the Top Online Psychiatric Services for Professionals?
- Seek Labs Unveils Investigational, Equipment-Free Molecular Prototype for MTB on Rapid-Deploy SeekIt™ Platform
- Your Local Business Needs a Better Website – Here’s Why
- Former Pfizer Chief Scientific Officer Dr. Mikael Dolsten, Joins MC Sciences as Independent Chairman
- Body Vision Medical Expands Access to AI Tomo® with Major LungVision® Platform Upgrade
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook

Vitestro Unveils Aletta ™ | World’s 1st Autonomous Robotic Phlebotomy Device
Aletta is the first and only Autonomous Robotic Phlebotomy Device™ (ARPD™), a unique technology descriptor that establishes a new category of medical robotics dedicated to optimizing blood collection. As the exclusive ARPD™, Aletta sets the global industry standard for automated venous blood draws—enhancing clinical precision, safety, efficiency, and patient experience.

Patients Transmit More Than 15 Million Vitals Using Pylo Remote Monitoring Devices
Patients use Pylo remote monitoring devices to collect and securely transmit health data like blood pressure, weight, blood glucose, oxygen saturation, and pulse rate readings to their care teams. With this data, providers can identify potential health issues early and intervene promptly, preventing potential hospitalizations.
PathMaker Neurosystems Announces Issuance of 3 Patents
“We are pleased to see these latest patent issuances that protect PathMaker’s novel technology for the non-invasive treatment of ALS,” said Jake Maslow, J.D., Co-Founder and Chief Intellectual Property Officer at PathMaker. “These patents provide additional protection specific to the treatment of ALS that goes beyond the more than 40 issued patents we already have protecting our platform globally.”
Trinity Biotech Secures Additional Funding for Glucose Monitoring Tech
Trinity Biotech notes the additional liquidity will support both the continued development of their innovative continuous glucose monitoring (CGM) technology and the Company’s comprehensive transformation plan.
Clinical Trials
Editas Medicine Reports New Safety and Efficacy Data from the RUBY Trial of Reni-cel in 18 Patients with Sickle Cell Disease | Presented at the European Hematology Association (EHA) Annual Congress
“These data confirm the observations from our prior clinical readouts and further support our belief that reni-cel has the potential to be a best-in-class and clinically differentiated, one-time, durable medicine that can provide life-changing clinical benefits to patients,” said Baisong Mei, M.D., Ph.D., Chief Medical Officer, Editas Medicine.
Innovent Biologics Delivers Oral Presentation on Clinical Data of IBI363 (First-in-class PD-1/IL-2α-bias Bispecific Antibody Fusion Protein) in Advanced Non-small Cell Lung Cancer and Other Solid Tumors at the 2024 ESMO Virtual Plenary
Dr. Hui Zhou, Senior Vice President of Innovent, stated: “IBI363, as a first-in-class molecule, represents Innovent’s continuous innovation and advancement in the immunotherapy field. Starting from molecular design, the unique approach of α bias with β and γ attenuated were creatively adopted, which greatly improved the therapeutic window of IL-2. Meanwhile, through the specific traction of PD-1, tumor-specific T cells expressing both PD-1 and CD25 can be selectively stimulated and amplified, thus exerting anti-tumor effects. IBI363 has demonstrated excellent druggability with antibody-like pharmacokinetics (IgG-like PK) and low immunogenicity.

Positive 6 Month Results from CroíValve’s TANDEM I Study to Treat Tricuspid Regurgitation Presented at New York Valves 2024
Favorable patient outcomes from the TANDEM I first-in-human clinical trial of the CroíValve DUO™ System for the percutaneous treatment of tricuspid regurgitation (TR) were presented today by Professor Wojciech Wojakowski at the New York Valves 2024 scientific conference.
Biotechnology News
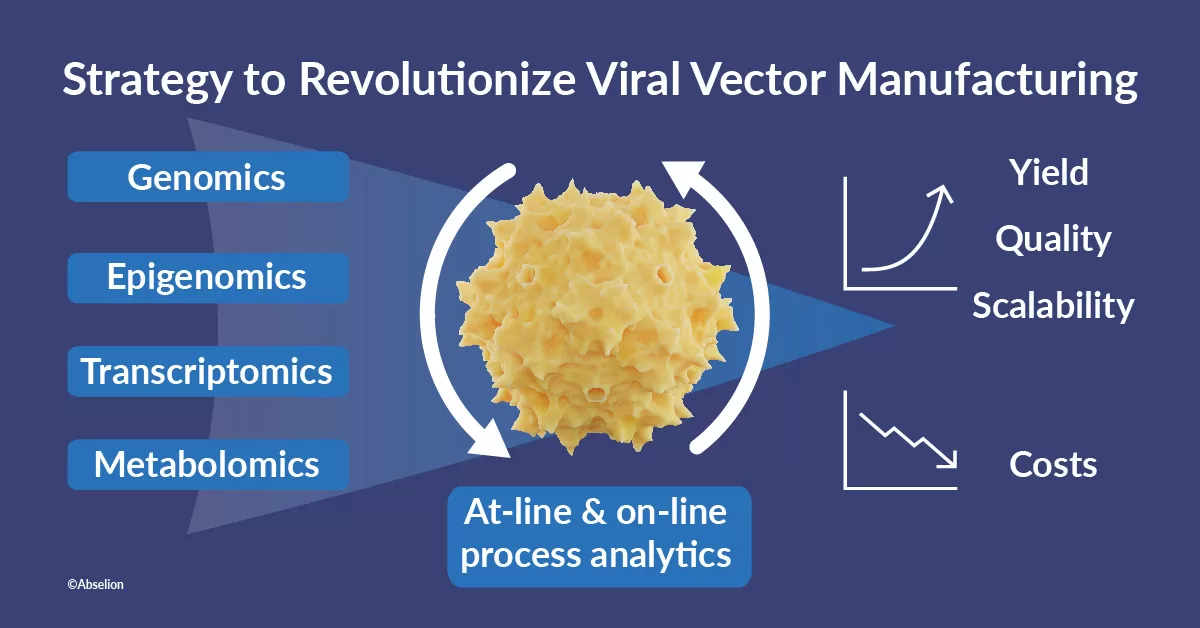
International Consortium to Revolutionize Viral Vector Manufacturing | Reports VVector Bio
“This project represents a paradigm shift towards improving viral vector manufacturing yields,” remarked Alina Venereo Sanchez, CEO of VVector Bio. “By integrating genomics, epigenomics, transcriptomics, metabolomics and new ways of directly integrating analytics into production, we’re poised to unlock unprecedented production capabilities.”

Qubit Pharmaceuticals nominated for “Best start-up 2024” award by Prix Galien USA
Qubit Pharmaceuticals has been nominated for the 2024 edition of the prestigious Prix Galien USA, in the “Best Startup” category, out of a field of 41 companies, including 15 French contenders.
TEPKINLY (epcoritamab) Receives Second European Commission Approval for the Treatment of Adults with Relapsed/Refractory Follicular Lymphoma
TEPKINLY is the first and only subcutaneous bispecific antibody approved as a monotherapy in the European Union to treat both relapsed or refractory (R/R) follicular lymphoma (FL) and R/R diffuse large B-cell lymphoma (DLBCL), after two or more lines of systemic therapy.
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Viraj Gandhi On Automation and Technology – to Drive Progress in Pharmaceutical Manufacturing
This article takes a closer look at the key benefits of automation and technology in pharmaceutical manufacturing and how Medivant Healthcare harnesses these solutions to drive progress across our production facilities. Read what Viraj Gandhi has to say.

Limb Loss and Preservation Registry (LLPR) Transforms Care Through Data and Insights | By Shawn Murphy, Vice President, Thought Leadership & Innovation Foundation (TLI)
Shawn Murphy writes, “The Limb Loss and Preservation Registry (LLPR) represents a pivotal development in patient care. It stands as the first collaborative database that unites hospital and health systems, provider organizations such as Accountable Care Organizations (ACOs), Integrated Delivery Networks (IDNs) and orthotic/prosthetic (O&P) practices, focusing on both upper and lower extremity acquired and congenital limb differences, as well as limb preservation populations. This collective effort has the potential to drive substantial advancements in patient outcomes, treatment effectiveness and care quality.” Read to learn more.

It Takes an Ecosystem – Bringing Stakeholders Together is a Critical 1st Step to Solving Problems In Healthcare | By Andrew Cleeland, CEO, Fogarty Innovation
Andrew Cleeland writes, “Introducing a new medical therapy or technology is a complex, expensive, and time-consuming journey, one that is fraught with significant risk. While innovation often starts with a clear, well-defined unmet clinical need, it must be paired with an equally compelling value proposition. My mentor, Dr. Thomas Fogarty, once said, “An idea, by itself, has no importance whatsoever; it is the implementation of that idea and its acceptance by others that brings benefit to our patients.” Read on.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
Palatin Technologies Announces $5 Million Registered Direct Offering
Palatin Technologies is a biopharmaceutical company developing first-in-class medicines based on molecules that modulate the activity of the melanocortin receptor systems, with targeted, receptor-specific product candidates for the treatment of diseases with significant unmet medical need and commercial potential. Palatin’s strategy is to develop products and then form marketing collaborations with industry leaders to maximize their commercial potential.
CIONIC Raises $12 Million Series A Extension Led by L Catterton with New Partners THVC and Enable Ventures
Nine months after the start of shipments, Cionic Neural Sleeve licensed nearly nationwide with more than 200,000 hours of customer use, transforming the lives of those with mobility impairments.The company expands the board with the addition of Whitney Casey, Venture Partner at L Catterton.
Akura Medical, a Shifamed Portfolio Company, Closes $35M in Series B Financing
Akura Medical reports the funds will be used to apply for FDA 510(k) clearance for the Akura Mechanical Thrombectomy Platform, support clinical trials for additional indications, and scale manufacturing capabilities.

Laverock Therapeutics Raises £13.5M
Laverock Therapeutics notes the funding will enable further development of the GEiGS technology and progression of Laverock’s programs in regenerative medicine and immuno-oncology, with a focus on Type I Diabetes and solid tumor responsive T-cell and macrophage-based immune therapies, through to in vitro and in vivo validation.
Market Reports
Advancements In Imaging
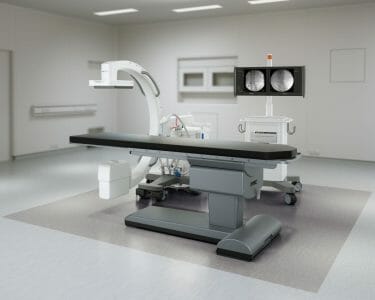
Philips Extends Company’s Mobile C-arm Portfolio with Zenition 10
Based on Philips’ state-of-the-art flat panel detector technology, Zenition 10 helps to expand patient access to routine surgical care and minimally invasive procedures.

Mediso Launches Next Generation MRI Spectrometer spinScan®
Mediso notes the spinScan® spectrometer is based on a technology that has been continuously developed for 10+ years, featuring an FPGA SoC platform with highly scalable transceiver architecture enabling more than 128 receiver channels and microsecond gradient resolution with closed-loop control via high-speed optical interfaces
Simon Hegele Healthcare Solutions Accelerates Global Growth Through Partnership with Brazil’s MR In Site
“Our partnership with MR In Site is a great opportunity to expand our capabilities in the South American market,” says Jim Nestel, CEO of the Americas and Asia Pacific for Simon Hegele Healthcare Solutions.
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications

Inclusive Design: How To Incorporate Accessibility When Creating a Healthcare Website
Accessibility has become a crucial part of website development. This helps provide seamless interactions for everyone, regardless of their abilities or disabilities. The healthcare industry,

Exploring the Benefits of Outsourcing Medical Billing: 8 Tips for Vendor Selection
In the present-day healthcare realm, medical professionals encounter countless trials, one of which pertains to effectively handling medical billing procedures. Healthcare providers, be they big

Evaluating Key Performance Indicators in Medical Billing: 9 Tips for Performance Analysis
In today’s rapidly evolving healthcare industry, efficient medical billing is essential for the long-term financial stability of any medical practice or institution. Not only do
Exploring the Role of Communication in Effective Healthcare
Communication in healthcare goes beyond exchanging information. It serves as a vital link between healthcare professionals, patients, families, and interdisciplinary teams.
Healthcare Careers
Nurses Corner

How Can You Advance Your Nursing Career
Getting a job is only the beginning of building a career. After getting the correct position, you must also focus on growing as an individual
Parker Health Group Wins 2022 McKnight’s Tech Awards in Skilled Nursing
Parker Health Group was chosen as the Gold winner for achieving overall hand-hygiene compliance at or above 95%, staff time savings and efficiency versus direct observation, and positive feedback from care partners and family members for commitment to safety and quality for the last 17 months. These outcomes also improved resident, family, and care partner satisfaction and experience.

4 Most-In Demand Nursing Jobs
In the last decade, demand for qualified nurses has been increasing, and the pressure of a global pandemic on healthcare systems has exacerbated the nursing

