- Intensity Therapeutics, Inc. Announces $2.35 Million Public Offering
- Carr Tech Corp Celebrates FDA 510(k) Class II Approval of FROG All-in-One Filter Needle
- Veracyte Announces Expanded Availability of Decipher Prostate Test to Patients with Metastatic Prostate Cancer
- Second Phase of ADVANTAGE AF study of FARAPULSE ™ Pulsed Field Ablation System Meets Primary Safety and Efficacy Endpoints
- Overture Life Secures $57M in Total Funding | Earns U.S. CLIA License to Deliver Reliable, Scalable IVF Automation
- CorWave Completes 6-Month In Vivo Study for Clinical Trials Initiation
- Vektor Medical Announces Post-Market Study Evaluating Benefit of AI-Powered Analysis to Identify and Ablate Non-Pulmonary Vein AF Targets
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
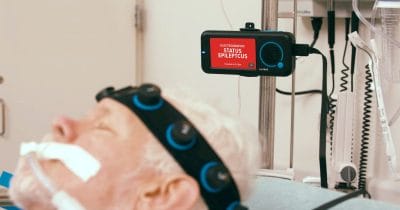
Ceribell Launches ClarityPro ™ AI, Expanding Access to Critical Diagnosis of Electrographic Status Epilepticus
ClarityPro is an AI-based software algorithm within the Ceribell EEG system that analyzes EEG waveforms to diagnose electrographic status epilepticus (ESE). It is the first acute care device to be FDA indicated for stand-alone diagnosis.
Medical Equipment Upgrades in the Dental Space: Revolutionizing Patient Care
In this article, we will explore the latest innovations in the dental space and delve into the importance of autoclaves in maintaining a safe and sterile environment.
Study Organized by Main Line Health Researchers Reverses FDA Warning on Vital Peripheral Artery Disease Treatment
Results of the study on devices coated with paclitaxel were presented today at the TCT 2023 meeting in San Francisco, the world’s largest interventional cardiology meeting. The study shows no increased risk of death from use of paclitaxel-coated devices and has simultaneously been published on a fast-track basis by The Lancet.

PeekMed Expands into the US with a New Subsidiary and New Product
The decision to form PeekMed LLC is driven by a desire to strengthen presence in the world’s largest and most influential healthcare market. This expansion will enable PeekMed to offer versatile solutions and provide timely support to its growing customer base in the US.
Clinical Trials
Neoadjuvant Study with LTX-315 In Early-stage Melanoma Patients to be Presented at the 15th Nordic Melanoma Meeting
The title of the presentation is: Neoadjuvant LTX-315 in combination with pembrolizumab in resectable stage III/IV melanoma (NeoLIPA trial): Protocol for a single center phase II open label study.
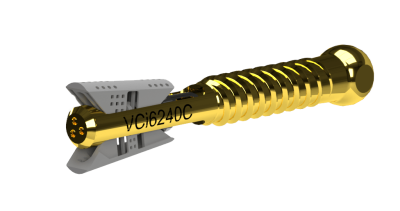
Amber Implants Announces Start of Clinical Trial with VCFix® Spinal System
This first-in-human clinical trial will assess the safety and effectiveness of the VCFix® Spinal System implant for patients suffering from vertebral compression fractures. The implant is provided with a user-friendly, single-use sterile surgical kit, ensuring perfect traceability and reducing the risk of infection.
Theradaptive Awarded DOD Clinical Trial Award of up to $7.4 Million
Commenting on the award, Theradaptive CEO and founder, Luis Alvarez, Ph.D. said, “The CDMRP Clinical Trial Award comes at a pivotal time for our company as we prepare for first in human clinical trials. Veterans and service members are disproportionally likely to suffer from traumatic extremity injuries or spinal degeneration and disc injury, and currently lack efficacious treatments with very few options. We have already demonstrated superior outcomes in preclinical studies, showing the promise of our technology. With this CTA award we are one step closer to providing new and improved therapies to patients and aligning with CDMRP’s strategic goal of trying to increase post-injury quality of life by halting or slowing orthopedic disease progression in servicemembers and veterans.”
Biotechnology News
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Cybersecurity, Medical Devices, and Recall Risk By Chris Harvey, Senior VP of Brand Protection at Sedgwick
“Connectivity can be a huge benefit if it means getting information to a doctor more efficiently, being able to monitor vital signs, or even having two different medical devices talk to each other to help manage care. But as with most technology applications, those benefits also make devices more vulnerable to cyber threats,” writes Mr. Harvey. Read more on what he has to say.

Transforming Pharmacy with Paperless Prescriptions By Nadeem Sarwar, Founder & CEO at Phlo Connect
Mr. Sarwar writes, “Our solution allows clinicians to generate and ‘sign’ a digital prescription, and then send this to the Phlo digital pharmacy, which can deliver the medication to the patient’s door in a timeframe that suits them. This ultimately enables a trackable, digital prescribing journey from the point of prescription to delivery.”
Announcing the Amazing Moby Cutter™. The Moby Is One of the Best Surgical Wire Cutters on the Market Today!
Moby Cutter ™. It is the first cutter of its kind, with a catchment device. This device can prevent cut pieces of material from becoming dangerous projectiles, which can puncture skin, endanger eyes or fall into the deep surgical field. It has been cleared for commercialization per FDA guidelines and sold as a prescription only (Rx) medical device.
Executives on the Move
FDA Medical Device Updates
Comprehensive Insights into Medical Device & Biotechnology Company Mergers, Acquisitions & Funding
9T Labs Raises $17M Series A Funding
9T Labs notes the investment will help fully commercialize the company’s Red Series® software tools, 3D printer, and molding equipment to lead the way in the production of sustainable high-performance parts for end-use applications.
EarliTec Dx Announces $19.5 Million Financing to Advance Autism Diagnosis and Treatment in Children as Young as 16 Months of Age
The EarliPoint® Evaluation for Autism Spectrum Disorder is an investigational device utilizing Dynamic Quantification of Social-Visual Engagement (DQSVE), to capture moment-by-moment looking behavior imperceptible to the human eye.
Epitel Secures $12.5 Million Series A Financing for Wearable, Wireless EEG Monitoring System
Epitel, Inc., a digital health company developing a wearable, wireless EEG monitoring platform for seizure detection, announced today the closing of a $12.5 million Series
Variantyx Secures $41.5M in Funding
“The funding is a great testament of confidence and trust in the company,” said Haim Neerman, CEO of Variantyx. “It allows us to expand our diagnostic solutions to a wider array of customers so that medical care can become more personalized. The growth of Variantyx has been exponential, and we look forward to sharing the new disruptive products we have in the pipeline.”
Market Reports
Advancements In Imaging
Hospitals In the News
Medical Device and Biotechnology Executives: Innovative Professionals on the Move and Making Waves in the Industry
Non-Profit News
Healthcare: Understanding the Business Side of the Industry and Its Implications
Helpful Tips For Picking Medical Equipment For Your Business
Read this article now and learn how to pick out medical equipment for your start-up!

How Can SAST Be So Much Useful?
Software development could easily open up the door to cybercriminals. This is why applications must contend with a constant barrage of malicious activity from bots

Equipment That Will Improve The Quality Of Your Healthcare Services
Providing quality healthcare services is important for any medical practice. Whether you are a doctor, nurse, or other health care professional, having the right equipment
How Healthcare Providers Can Embrace A Data-Centric Approach
Data is an integral part of business operations in all verticals, and healthcare is no exception. Leveraging patient information helps hospitals and clinics to provide





