- Innovaderm Research Rebrands as Indero | a Dual-Focus CRO
- Abbas Kazimi Steps Up as Dynamic CEO of Nimbus Therapeutics
- Reflow Medical Expands Global Reach with New European Subsidiary in Landsberg am Lech, Germany
- COEPTIS’ NexGenAI Affiliates Partners with NUBURU Network to Drive Innovation in AI and Robotics as Part of its Transformation Plan
- Mirva Ekman Appointed Quality Director & Member of the Management Team at Bioretec
- Vitestro Unveils Aletta ™ | World’s 1st Autonomous Robotic Phlebotomy Device
- Phantom Neuro Receives FDA Breakthrough Device & TAP Designations, Solidifying Position as a Neurotech Leader
Medical Device News Magazine delivers the latest updates from the medical device and biotechnology sectors.
Stay Ahead in Medical Technology
At Medical Device News Magazine, we take pride in delivering high-quality, in-depth news. Our content goes beyond the surface, providing a comprehensive look at the trends, innovations, and breakthroughs that are shaping the future of medical technology.
In addition, we aim to offer a unique perspective on the rapidly evolving healthcare landscape. By following our news, you gain exclusive insights into industry developments, emerging technologies, and regulatory updates that impact medical devices worldwide. At the same time, you become part of a vibrant community of professionals, innovators, and thought leaders who are dedicated to advancing healthcare through cutting-edge solutions.
Furthermore, we encourage you to explore our diverse range of content, from the latest product announcements to in-depth analyses of industry trends. If our vision aligns with your objectives, we also offer opportunities for advertising and collaboration, allowing you to reach a highly engaged audience of medical technology professionals.
Stay informed, stay connected, and join us as we navigate the future of healthcare together.
Medical Device Industry News: Latest Developments, Breakthroughs, and Future Outlook
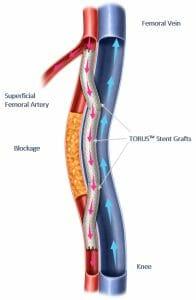
Endologix Receives FDA Approval of the DETOUR System to Treat Long Complex Superficial Femoropopliteal Lesions in Patients with PAD
“We are delighted to receive FDA approval of the DETOUR System,” said Matt Thompson, MD, President, and CEO of Endologix. “PTAB therapy represents a significant step forward for patients with complex PAD, they have long needed a more effective and less invasive treatment option for long lesions of the SFA. We are proud to be pioneering this novel approach and continuing to innovate on behalf of patients. We look forward to launching this new therapy in the U.S. through a targeted market release in the coming weeks.”
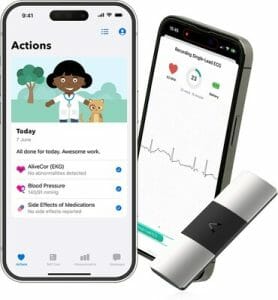
AliveCor Collaborates with European Market Leader in Remote Patient Monitoring Luscii to Offer World’s First ‘Virtual Heart Clinic in a Box’
Together, the companies will launch the world’s first ‘virtual heart clinic in a box’, revolutionising cardiac care for millions of patients by making it easy for any hospital or GP to deliver high-quality, remote patient monitoring to their patients.
Magenta Medical Reports 1st Patients Treated in the US with the World’s Smallest Heart Pump
“Magenta Medical CEO Dr. David Israeli, CEO said, “Magenta is proud to partner with top cardiology centers in the United States to further the validation of its technology and provide cardiologists with a powerful tool to support their high-risk patients during complex procedures. We are looking forward to a speedy recruitment process and favorable study outcomes that would allow us to take the next step in the clinical program towards ultimate market approval.”

Laird Thermal Systems Unveils Micro Multistage Thermoelectric Cooler & Optical TEAs Integration Capability
“Advanced process automation has enabled a new level of miniaturization for our MSX multistage series to support next-generation imaging solutions that require smaller optical packages,” said Andrew Dereka, Thermoelectrics Product Director at Laird Thermal Systems
Clinical Trials
Stanford Spinoff PhysioWave Announces Clinical Study with Omron Healthcare
The PhysioWave Pro™ scale measures the stiffness of the major blood vessel into which the heart pumps, the aorta. Stiffness increases with age, but excess stiffness among people of similar ages can be a warning sign of risk. It is established that increased vascular stiffness can predict the later development of high blood pressure, or hypertension, and a higher risk of cardiovascular disease.
MedAlliance Enrolls First US Patient into its Third FDA IDE Study: SFA SELUTION4SFA
SFA SELUTION4SFA is being conducted in over 30 centers in the US plus an additional 10 centers worldwide. The study will enroll 300 patients, with the aim of demonstrating the superiority of SELUTION SLR over balloon angioplasty (POBA). The primary efficacy endpoint is primary patency of the target lesion at 12 months and the primary safety endpoint is freedom from death at 30 days. Enrollment into the study will be complete by the end of 2023.
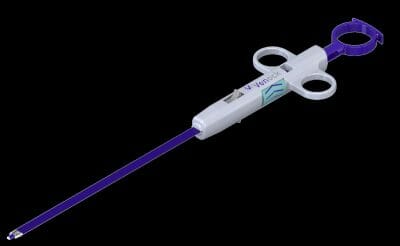
Economic Study Shows Significant Cost Savings when Using Venock Device for Large Bore Venous Access Site Closure
According to a new study, this device could provide cost savings of more than $1,700 per procedure. In addition, the device will enable same-day patient discharge providing a cost savings of up to $6,290 per patient. The Venock device replaces the tedious and lengthy manual compression steps currently used to close venous access sites after a catheterization – a decades old procedure that is extremely burdensome to patients. Medical approval of the device is forthcoming.
Biotechnology News
Our Experts – Byline Articles
Read Their Views & Share With a Colleague or Two!

Why Medical Devices Fail and What You Can Do About It
Medical devices fail for a number of reasons, but the consequences can be dire for patients and their doctors. Read to learn how.
5 Things a Laboratory Information Management System Can Do To Boost Productivity
Laboratory Information Management System can greatly boost productivity in your labs. Read on to learn more.

New Hope for Advanced Heart Failure: The Total Artificial Heart By Thomas A. Vassiliades, MD MBA
Thomas A. Vassiliades, MD MBA writes, “BiVACOR is developing the Total Artificial Heart, the first long-term therapy for patients with advanced heart failure. The TAH is an implantable rotary biventricular blood pump that uses magnetic levitation technology to replace both ventricles of a failing heart.”
Read his article to learn more!
Executives on the Move